Global Commercial Presence
Global Reach. Local Impact.
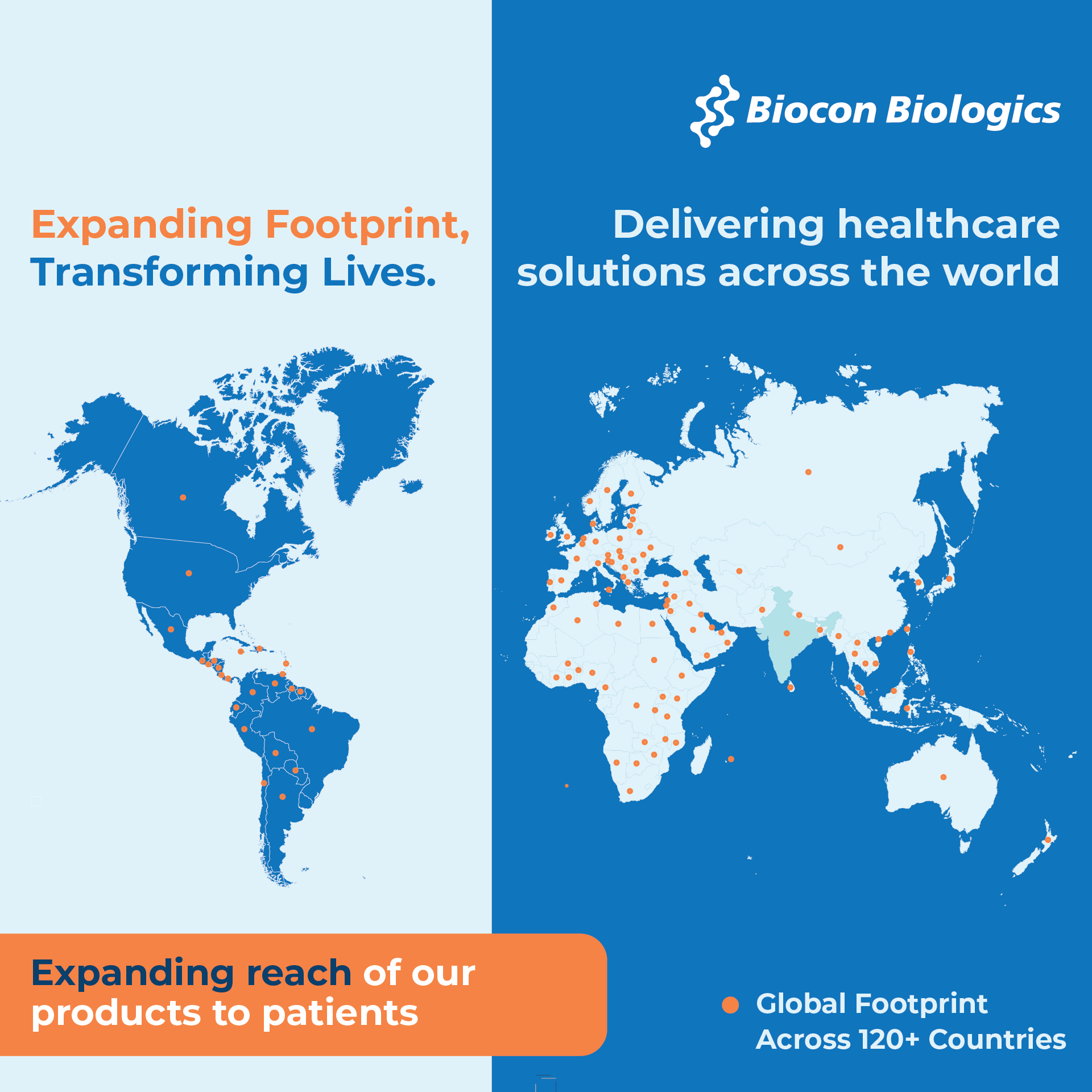
At Biocon Biologics, our purpose is to deliver affordable, life-changing medicines to patients across the globe. With a global footprint spanning over 120 countries, we are expanding access to high-quality biosimilars—bringing hope and health to patients worldwide.
Following the successful acquisition of Viatris’ biosimilars business in 2022, Biocon Biologics accelerated its transformation into a global commercial organization—building a strong on-the-ground presence across 29 self-led markets. These include the U.S., Canada, Germany, France, the UK, Spain, Italy and 14 other European countries, as well as the UAE, Saudi Arabia, Morocco, South Africa, Brazil, Malaysia, Thailand, and the Philippines.
Beyond these, we collaborate with a trusted network of strategic partners and distributors, extending access to our biosimilars to patients in many more countries across the world.
Our wide, global commercial footprint reflects our commitment to access to ensure every patient, everywhere, can benefit from the promise of biologics.