Home > Science > Research & Development > Analytics and Bioanalytics
Analytical & Bioanalytical Sciences
Biocon Biologics is fully integrated to develop high-quality biosimilars, including recombinant insulins, monoclonal antibodies, and other protein therapeutics. Biosimilars are closely matched versions of original biologics, conceptually similar to generics in chemically synthesized drugs. Since biosimilars are produced in living organisms, some structural or compositional variability is expected, requiring rigorous characterization of physical, chemical, and biological properties to ensure safety, efficacy, and consistent product outcomes.
To meet global regulatory standards and assure patient safety, biosimilars must demonstrate structural, functional, and clinical comparability with the original biologic, showing no meaningful differences in clinical outcomes. Biocon Biologics has invested in advanced technologies and a team of expert scientists, whose in-depth analysis and characterization studies ensure the highest quality and consistency of biosimilars throughout development, approval, commercialization and complete life cycle management.
Establishing Biosimilarity
Our R&D strength in biosimilar and biologics development is focused on a strategic QbD driven approach orthogonal techniques and fingerprint similarity.
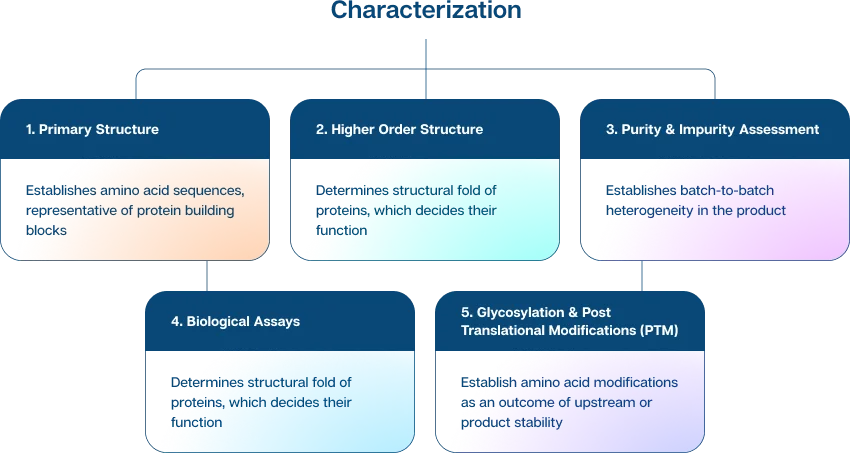
Functional & Physicochemical Characterization
A multitude of assays, across a spectrum of molecules, is used to assess product structures and undertake functional characterization. Orthogonal technologies and suitable analytical methods are applied to in-process and batch-release tests. We also use sensitive methods to assess pharmacokinetics and immunogenicity to ensure global standards of safety and efficacy.
- Potency Assays for insulins, peptides, MAb and MAb conjugates
- Immunology-based functional assays including T cell proliferation assays
- Tumor cell-immune cell-culture assays
- Development and validation using surface plasmon resonance, radioligand binding and flow cytometry
- Proliferation and cytokine evaluation assays
- Development, qualification and valuation of PK and immunogenicity measures, using ELISA and ECL formats.
- Cell-based potency assays for neutralizing antibody determination
Functional & Physicochemical Characterization
Analytical technologies are important to gain insights into the biological products’ structure, composition and properties.
Physicochemical characterization, for structural and compositional attributes
Functional characterization, for the product’s effectiveness (how the biological product binds to the target, and its biological outcome)