
Home > Science > Research & Development > Clinical Development
Clinical Development
Clinical Development
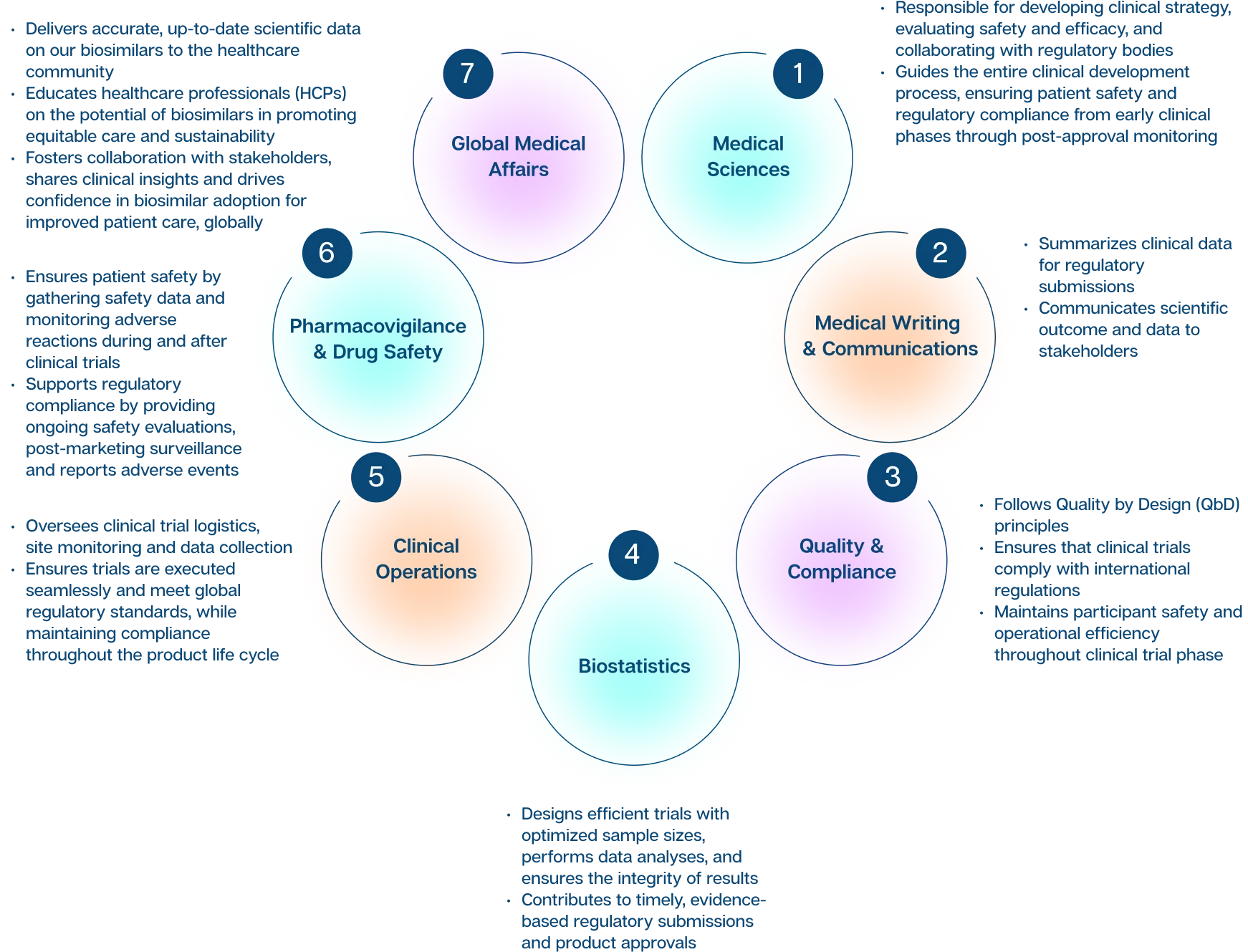
Global Medical Affairs
The Global Medical Affairs team bridges the gap between Biocon Biologics and the healthcare community by delivering accurate, up-to-date scientific data on our biosimilars.
The team is responsible for educating healthcare professionals (HCPs) on the potential of biosimilars to promote equitable care and sustainability, playing a pivotal role in advancing the scientific and clinical understanding of our biosimilars and biologics portfolio. In addition to supporting the development of clinical guidelines in close collaboration with regulatory bodies, it leads key medical education initiatives, including webinars and scientific conferences, to foster knowledge exchange and drive innovation.
Through active engagement with Key Opinion Leaders (KOLs) and stakeholders, Global Medical Affairs facilitates collaboration, shares clinical insights, and drives confidence in the adoption of biosimilars, for improved patient care.

