
Home > Science > Research & Development > Regulatory Sciences
Regulatory Sciences
This division plays a critical role in supporting the successful development and global commercialization of biosimilars. The team’s active participation and engagement with various functions across the organization, including R&D, Manufacturing, Quality, Commercial and Supply Chain, have established Biocon Biologics as a pioneer in biosimilar approvals.
Regulatory Affairs: Key Functions
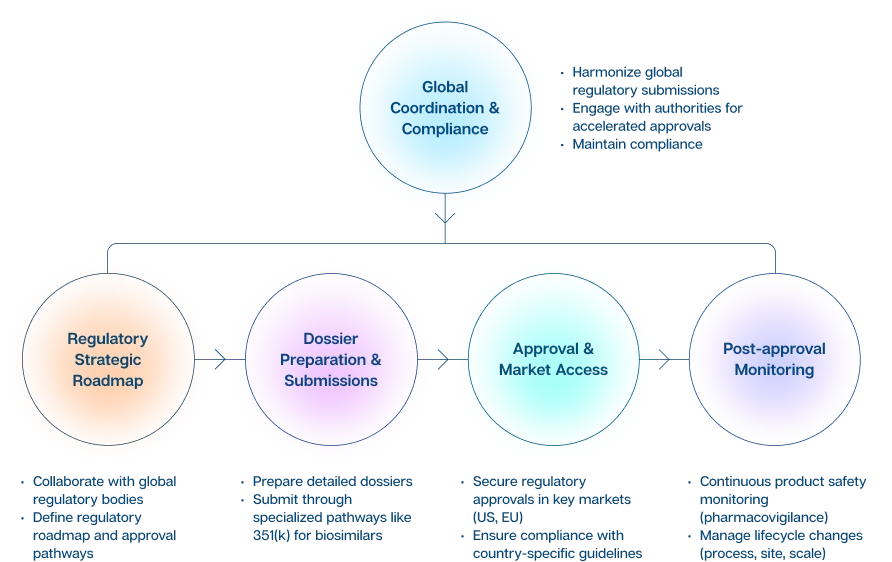
This team is pivotal in guiding our biosimilars strategy for market launch, as well as ensuring that products meet international standards and regulatory requirements throughout their lifecycle. Successfully navigating complex, country-specific regulatory pathways, and interfacing directly with regulatory authorities, including the U.S. FDA, EMA, MHRA (UK), TGA (Australia), Health Canada, ANVISA (Brazil), and PMDA (Japan), Regulatory Affairs has a differentiated strategy that has been driving Biocon Biologics’ expanding commercial footprint.
Biocon Biologics has achieved many global ‘firsts’ and set new benchmarks for the global biosimilars industry.
First Indian company to introduce a biosimilar (bGlargine) in Japan’s highly regulated market, in 2016.
First company globally to receive U.S. FDA approval for biosimilar Trastuzumab in 2017 and biosimilar Pegfilgrastim in 2018.
First company globally to commercialize interchangeable bGlargine in the U.S.
First company to get U.S. FDA approval for interchangeable bAflibercept.

